Perfect Tips About How To Draw Valence Electrons

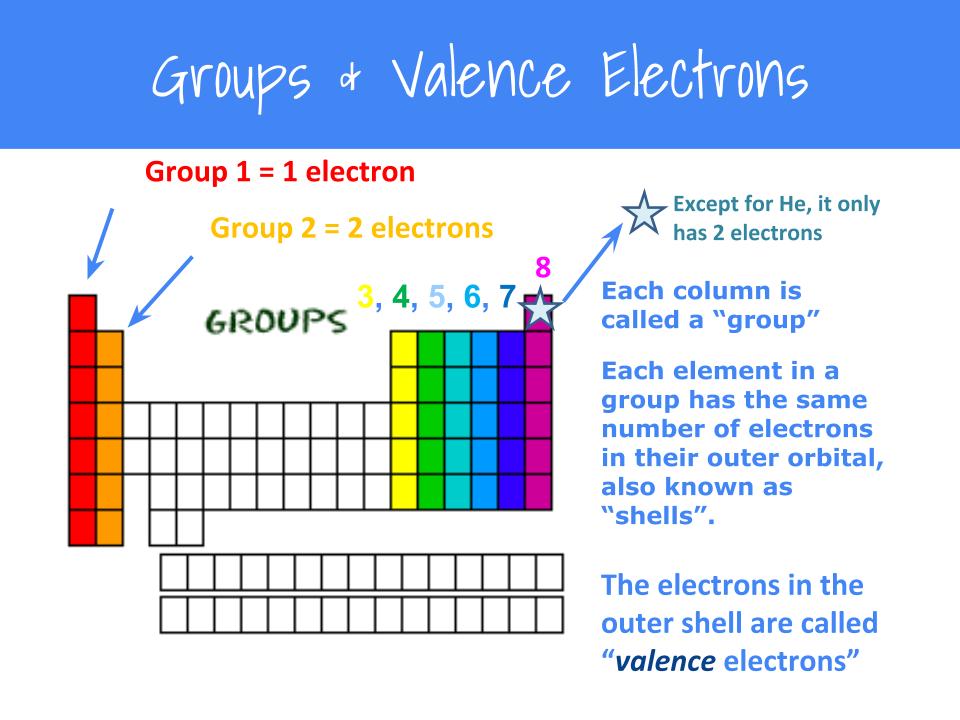
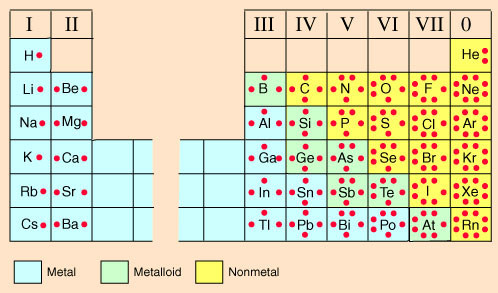
To find the valence electrons, look at the atom’s main group number.
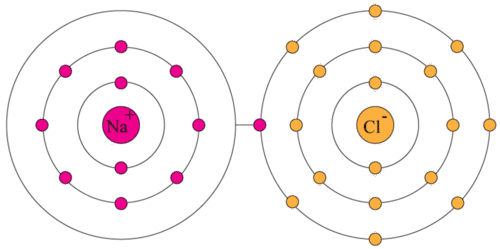
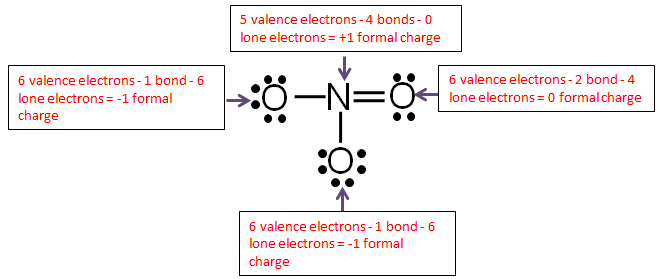
How to draw valence electrons. They are the electrons available for bonding. Neon, with its configuration ending in \(2s^2 2p^6\),. Valence electron, any of the fundamental negatively charged particles in the outermost region of atoms that enters into the formation of chemical bonds.
In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Valence electrons, their variation in the periodic table and relation to reactivity and electrical conductivity of elements. For instance, the oxygen atom belongs to group 6, therefore, the number of valence electrons in oxygen is 6.
You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. Whatever the type of chemical bond. This video reviews how to determine the number of valence electrons in a main group element, how to draw a lewis dot diagram for an element and how to draw l.
For example, atoms in groups 1 and 2 have 1 and 2 valence.







/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)